Originally posted 8/2/21. NOTE from 2/14/22: At this point this post is very out of date, and I have not had a chance to update it for more recent research from Delta. Furthermore, Omicron is sufficiently different from Delta that we don't know if this research will apply to Omicron, and it's too soon to have good field data on the incidence of long COVID in Omicron cases.
Updated 8/11/21 with more information and better estimates on how we can personally and collectively control the spread on Delta, how vaccine effectiveness changes over time, much more evidence that long COVID could last many years, and the first hard data on how the severity of long COVID depends on the severity of the initial infection.
Key takeaways:
- The US will almost certainly have a big spike of Delta cases in mid August to September. Try to not get sick when everyone else is getting sick, as it will be harder to get good medical attention, and if you are in a less-vaccinated area, the hospitals may be full of mostly unvaccinated people sick with Delta.
- Delta is much more transmissible than original COVID, and somewhat deadlier (at least, for the unvaccinated).
- If you’re young, healthy, vaccinated, and catch Delta, you’ll likely have a relatively mild acute infection, and your chance of hospitalization or death will be very low. However, you’ll run a significant chance (possibly a ~2.5% chance, but this is a low-confidence estimate) of acquiring some long-term chronic condition (eg brain fog, decreased lung function, chronic fatigue, loss of smell or taste) that doesn’t go away for many years, perhaps for the rest of your life. These long term chronic conditions vary in their severity – of the people who got long COVID after a mild COVID infection, about 18% said that their long COVID limited their daily activities a lot. You’ll have to make your own decision on how hard you want to work to avoid this risk.
- Your protection from catching a Delta infection, and your risk of getting long COVID, depends substantially on how recently you were vaccinated, with some evidence of a gradual dropoff in protection more than 4 months after your second shot. This is why many governments are starting to look at 6-month booster shots. If you had a single-dose J&J shot, you'll want to get a single-shot mRNA booster now. It's backed up by safety and efficacy research, and many vaccination sites are now willing to do this.
- A vaccinated person who works to keep their risk of infection from Delta low has a ~1/6x chance of catching long COVID relative to an equivalently behaving unvaccinated person (again, this is a low-confidence estimate based on limited data).
- If you want to have a low chance of catching Delta, you’ll need to go back to minimizing indoor unmasked contact outside your household, including with vaccinated people, and wearing a good mask (N95 or better) in stores and other public settings. As before, if you do choose to socialize indoors, 1-on-1 hangouts are much safer than indoor parties or big dinners.
- Your choices can slow the spread of Delta in your social network and beyond. If you feel sick at all or find out you were exposed, avoid socializing for a few days. If you are sick, take a COVID test immediately. If you find out you were exposed, get tested 3-5 days after exposure. Keep avoiding socializing until symptoms disappear and you get a negative test result. Don’t be that person who gives your friend a lifelong chronic medical condition.
- There will probably be much better information in the next few weeks on the incidence of long COVID among vaccinated people who catch Delta. You may want to consider being extra cautious until we learn more.
- It’s hard to predict what things will look like a few months out. Other more immune-escaping variants might come out at any time and further change the landscape. Booster shots targeted at Delta and future variants are also coming in a few months.
What is the risk from Delta?
I’m still unsure how much I and other fully vaccinated people should worry about Delta, but I’m leaning towards taking it seriously based on the available early evidence.
First, Delta is *much* more infectious than the original COVID, with various groups estimating an R0 of 5-9 vs the original COVID’s 2-3. This means the current spike in Delta cases will require hard work to contain. While the US is struggling to stop exponential Delta case growth, many European countries are managing to keep Delta caseloads down through a combination of reinstating mask requirements and requiring vaccination for participation in public life. Using a mask in public areas and avoiding high risk scenarios will still reduce the viral load you’re exposed to, and if society takes these actions collectively we’ll slow the spread of Delta, which will prevent the medical system from being overwhelmed in less vaccinated areas. Increased infectiousness alone isn’t enough to make the disease of serious concern; there are plenty of mild illnesses like chicken pox with high infectiousness rates. What makes Delta a concern is the combination of high infectiousness with high risk of serious or ongoing illness.
How much does vaccination help against Delta infections?
Getting mRNA vaccinated is still a big benefit, both individually and societally. On average it drastically reduces the acute consequences of catching Delta, significantly reducing the severity of illness but often not preventing it entirely.
To dive in more deeply, when we think about the mRNA vaccines' impact on Delta, we care about two different aspects of protection:
- Protection against hospitalization is important because it affects the seriousness of Delta cases in vaccinated people.
- Protection against infection is important because it affects the rate at which Delta can spread in vaccinated populations. If protection against infection is low, Delta may grow exponentially even in a mostly-vaccinated group. This can be bad even if protection against hospitalization is high because so many people end up getting infected. It also affects how much we need to worry about long COVID, which we'll get to in the next section.
The good news is that protection against hospitalization is very strong! Delta infections tend to be much milder for fully vaccinated people, with recent data from Israel showing vaccinated people are 93% less likely to be hospitalized from Delta. If the pre-vaccination risk of hospitalization from COVID for someone in your age/risk group is 4%, then your post-vaccination risk of hospitalization if you catch Delta would be 4%*7%*(1/0.36) = 0.78%. (The additional 1/0.36 term is to prevent the 64% reduction in COVID cases that the vaccine provides from being counted, since we're answering the question of the risk of hospitalization presuming you get COVID.) Unless you’re older or in a high risk group, these are very good odds. Here’s one tool for estimating your pre-vaccination risk of hospitalization if you catch COVID based on age, sex, and underlying conditions; you can multiply it by 0.19 to get your post-vaccination risk.
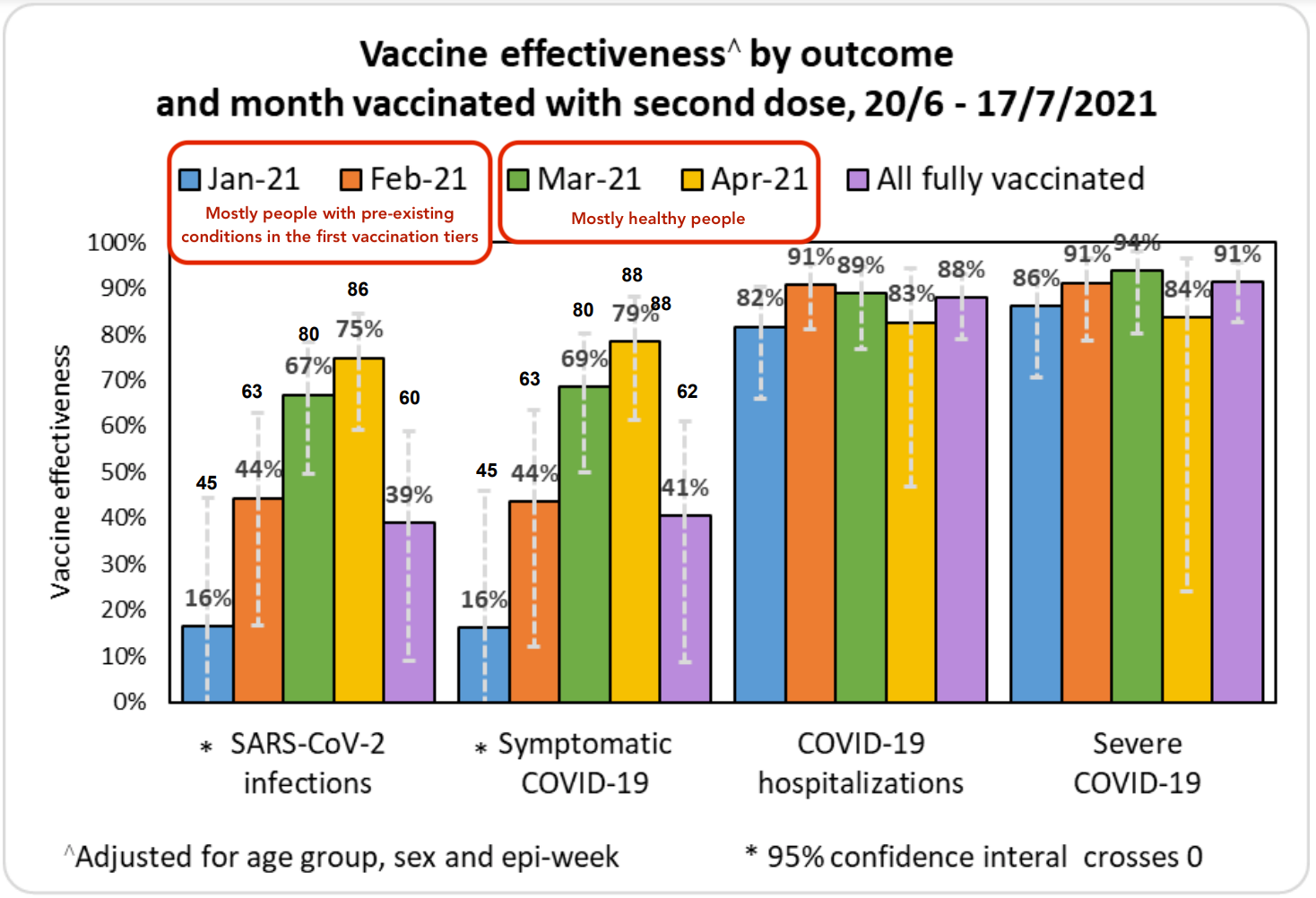
When we look at protection against infection, the data is more complicated. There’s new evidence that people who were vaccinated more recently are more protected from Delta because their antibody levels are higher. This is a strong argument in favor of giving everyone periodic booster shots, as getting full protection from the vaccine reduces the spread of Delta. The data from Israel below shows the vaccine providing strong protection against any Delta infection for about four months, but continued protection against serious cases requiring hospitalization for 6+ months.
However, Israel’s data might paint a needlessly pessimistic picture of protection agains infection as the people who were fully vaccinated in January and most of February tended to have pre-existing conditions. Israel didn’t open up vaccination to everyone until Feb 2nd, so this cohort started being fully vaccinated at the beginning of March. Thus, it’s primarily worth looking at the data from March onward to get a sense of how the vaccine is performing in the general population against Delta. While this only gives us visibility 4 months out, it’s better than nothing.
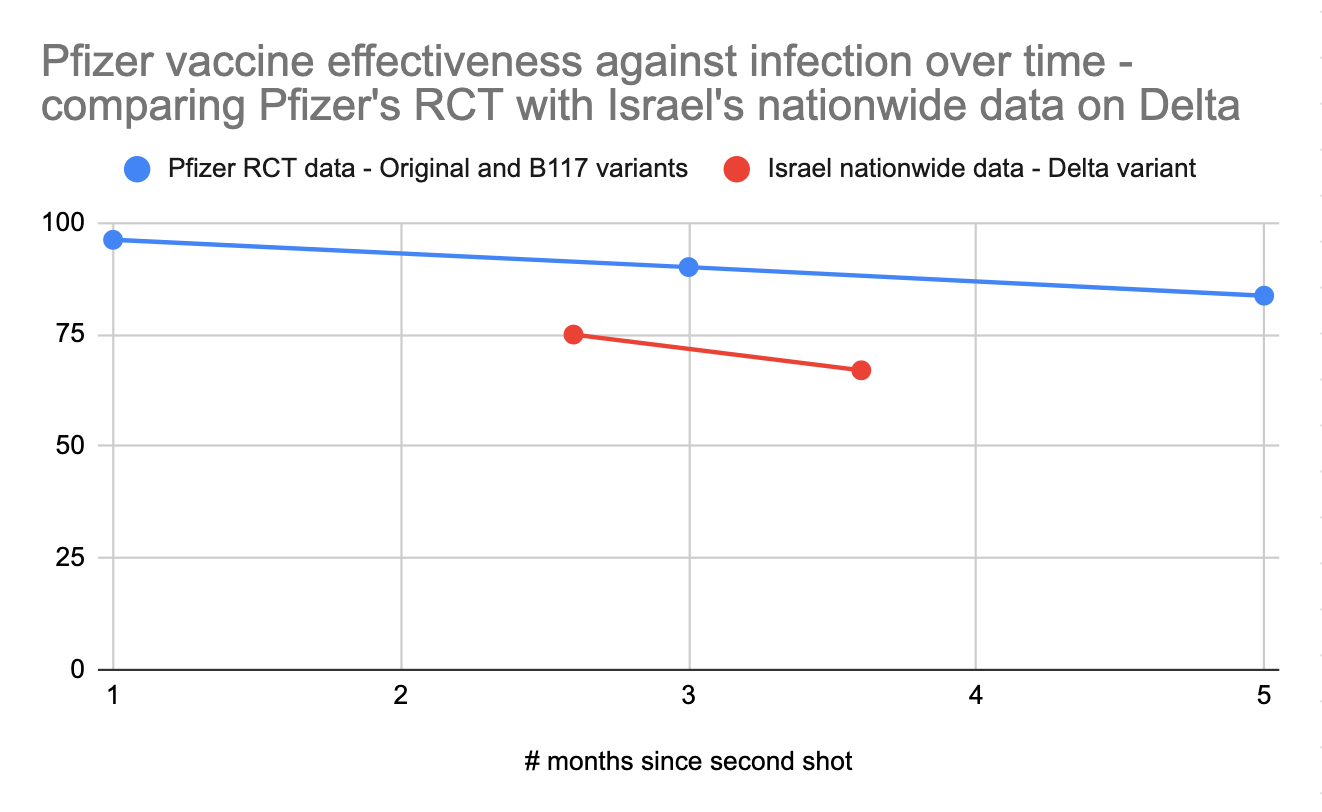
Pfizer’s official 6-month randomized controlled trial on ongoing vaccine effectiveness paints a more positive picture, with the vaccine retaining 83.7% efficacy against infection after 4 months after full vaccination as opposed to 96.2% efficacy during the first two months and 90.1% efficacy from 2-4 months. However, this study has a tiny sample size, with only 24 vaccinated people infected after 4 months vs Israel’s much larger nationwide dataset. Furthermore, the gathered data ends in March 2021, so it reflects performance against earlier variants, not Delta.
Moderna claims that their vaccine’s protection is undiminished after 6 months (93% for symptomatic cases) but they haven’t published the full research, and this data is also from before the Delta variant.
For these reasons, I’m inclined to primarily pay attention to Israel’s nationwide field data to get a sense of how mRNA vaccines fare against Delta, minus the data from Jan/Feb. What we can learn from the Pfizer study is that we should expect Pfizer's performance to gradually decline over 6 months in terms of its ability to prevent infections. If we look at the ratio between the protection against infection seen in the controlled Pfizer study against earlier variants vs the protection against infection seen in the field in Israel against Delta, and then project forward based on the decline in protection against infection over time, we could estimate that mRNA vaccines would offer a 40-60% protection against Delta infection 6 months after vaccination. This is of course an extrapolation based on limited data; we'll have the real answer from Israel in a few weeks.
As further evidence for the vaccines' declining effectiveness over time, we can compare data from Israel to other countries. Israel was the first country to undergo mass vaccination, which might explain why Israel’s numbers on the effectiveness of mRNA vaccines on Delta are somewhat worse than other countries that vaccinated more recently (eg England or Canada).
If we lived in a world with unlimited vaccines, everyone who was vaccinated early this year should go get a third shot to refresh their immune system's memory of how to fight COVID. Israel is starting to give high-risk groups booster shots, as these people were vaccinated over 6 months ago.
As things stand now, anyone who had the single-shot J&J vaccine should go get a booster Pfizer or Moderna shot now. This is supported by safety and efficacy research, and while some vaccination sites refuse to do this, others are actively soliciting people vaccinated with J&J to come in for an mRNA booster.
As a brief sidebar, if you or someone you know is still unvaccinated, know that Delta appears to have much higher viral loads and a somewhat higher hospitalization and mortality rate relative to original COVID for unvaccinated people, so it’s worth giving your body that practice fight against COVID prior to the real deal. Furthermore, since a full course of vaccination takes over a month to start working and Delta cases are skyrocketing, you’d better get started now; it’s very hard to outrun an exponential case growth curve.
Possible strategies, and the impact of long COVID:
Unless you’re willing to go back avoiding indoor dining and indoor social engagements with friends, there’s a good chance you’ll catch Delta, even if you’re only hanging out indoors with other fully vaccinated people. Delta is so infectious that it is spreading even in fully vaccinated groups, albeit more slowly than in unvaccinated groups.
Thus, there are two strategies to consider:
- F*ck it, Delta won’t be that bad because I’m fully vaccinated, let’s party!
- There’s still a lot we don’t know about Delta. It’s time to go back to being cautious and using tools like microcovid to minimize risk. I’m going to miss out on a lot of fun things.
Originally I was a solid (1), but now I’m leaning towards (2) in the short term. Here’s why:
Long COVID (aka Long-haul COVID, Persistent COVID Syndrome / PCS, Post-Acute COVID Syndrome / PACS), a set of persistent symptoms that never quite goes away, is surprisingly common, and can be seriously debilitating. Spending a week in bed from the flu is, long term, no big deal, but having permanent brain fog or diminished lung function definitely is, and it’s worth going to a big effort to avoid it. I know a handful of people of all ages who have long COVID, and it seems like a serious impact to productivity and quality of life, equivalent to losing a decade or more of lifespan. For younger people, the risk of long COVID is much more significant than the small chance of dying from an infection.
My longer term choice of (1) or (2) depends on some information we don’t have yet but will likely have within a couple of weeks, so I’m inclined to default to (2) until we have that information available. Specifically:
How much does being vaccinated reduce the risk of long COVID from the delta variant?
We can’t answer that question yet, but there are at least hints of an answer. We can do our best to extrapolate from previous long COVID studies, but Delta, which presents different acute symptoms from earlier variants, may be different enough that we can't make clear conclusions about long COVID from Delta. However, it's often better to have a rough guess than no information at all, as people will be making decisions about changes in behavior anyway.
We're going to extrapolate a risk of long COVID for vaccinated people by looking at the impact of the mRNA vaccines on disease severity for Delta, looking at the impact of disease severity on persistent long COVID, analyzing how much long COVID persists over time, and looking at other evidence that vaccination may reduce the incidence of long COVID.
Here’s what we know so far:
What is Long COVID?
Long COVID refers to COVID symptoms that persist or appear after the acute period of COVID symptoms is over. Some people have symptoms for a few weeks, others have symptoms that never go away.
It’s very likely that long COVID is actually multiple conditions lumped under a single label.
A recent clustering analysis of physical long COVID symptoms found two major clusters. The first cluster mainly exhibits respiratory symptoms, including difficulty breathing and chest pain. The second cluster mainly exhibits a constant state of tiredness.
Note that severity of the original infection only has a mild impact on the incidence of long COVID.
In parallel, the two leading theories for the cause of long COVID are (1) it’s the result of an autoimmune reaction triggered by COVID, and (2) it’s the result of the body never quite clearing the infection, perhaps because the virus is partially suppressing the immune system. Perhaps both are true, and the two theories map to the two symptom clusters. The first cluster could be an autoimmune disorder, with the body attacking lung tissues and other areas. The second cluster looks a lot like Chronic Fatigue Syndrome, which is still poorly understood by the medical community but thought to sometimes be caused by a persistent viral infection that the body can’t quite clear.
There’s evidence for both autoimmune reaction and a lingering infection. First, researchers recently found a large fraction of people with long COVID have autoantibodies, a particular type of antibodies associated with autoimmune disorders. Furthermore, people with asthma are at greatly increased risk of long COVID (more so than almost all other conditions), and asthma is an autoimmune disorder of the lungs, so it seems likely that asthma indicates a predisposition to autoimmune disorders, and that suggests people with autoimmune disorders may have an increased chance of an autoimmune-like long COVID. Furthermore, women are substantially more likely to get long COVID than men, and women in general are more likely to suffer from autoimmune disorders.
On the side of long COVID sometimes being a lingering infection, there is evidence that receiving vaccination after contracting long COVID helps ease symptoms, with 56.7% of members of a long COVID support group, surveyed retrospectively, reporting an improvement on symptoms (and 18.7% reporting a worsening of symptoms). There are obvious sampling bias issues with this study, but the positive effect of vaccination is likely still real, and perhaps even larger than the reported amount, since people who are cured from vaccination are presumably more likely to stop checking the support group. If long COVID is sometimes caused by a persistent viral infection, then it makes sense that providing the immune system with a large number of targets to ramp up the appropriate antibodies on would help it clear the remaining COVID infection.
In general, both autoimmune disorders and CFS tend to be difficult to treat, with a range of drugs and other interventions offering varying levels of amelioration of symptoms without a complete cure. The lack of clear solutions to these conditions makes the prospect of reliably curing long COVID more daunting.
There's also a theory that the more serious COVID infections are causing lasting physiological damage that then leads to long COVID. Thus, even if the infection is cleared and there's no ongoing autoimmune response, there's still scarring in the lungs and clotting damage in other parts of the body that causes ongoing symptoms. This is backed up by physiological evidence – X-rays of recovered severely ill COVID patients still show ongoing lung abnormalities. Physical scarring obviously is very difficult to treat as well.
How long is long COVID?
Obviously, we should care a lot more about long COVID if it's a lifelong affliction than if it gets better after a few months. However, since COVID has only been around for about 18 months, we don't have any direct data to go on around lifetime impact. We can solve this by looking at two sources of evidence:
- Looking at data on long COVID over a shorter timeframe, and seeing what we can do to extrapolate forward
- Looking at long-term data from other coronaviruses such as the ones that caused the SARS and MERS epidemics, as well as other similar diseases, and assuming this will give us some hints about what will happen with COVID
First, we'll look at the thicket of long COVID studies out there. Diving into long COVID has been tricky and confusing, partly because it's been measured in very different ways, and partly because it manifests so differently in different people. It's tough to do a risk analysis when some studies report the incidence of long COVID as low as ~2% and others report it as high as 1 in 3. In general most experts appear to quote long COVID rates as occurring in 10-30% of COVID cases when talking to the media.
In part these differences between studies are driven by factors like:
- How information on symptoms is gathered – some use self-reporting via an app, while others use medical records.
- How patients were found, and whether asymptomatic COVID patients were included.
- Definitions of long COVID – some count any symptoms after 4+ weeks as long COVID, while others count only 12+ weeks.
- Sample size – Studies vary from under 100 patients to half a million.
- Whether any attempt was made to distinguish whether new symptoms were from COVID or another cause. Nearly all the studies were observational and had no control group.
So far my favorite source on this information is a UK study that actually has a control group and prospectively and repeatedly surveys a random sample of people in conjunction with providing regular COVID tests so that it actually catches asymptomatic cases. This study was conducted prior to vaccination and prior to the dominance of the delta variant.
Here’s the percent of people who catch COVID who are still experiencing at least one symptom, by day after infection:
From the above graph, we see that, if we subtract out the frequency of symptoms shown in the control group, roughly 55% of COVID patients who had symptoms at 5 weeks still had at least one symptom roughly 4.5 months, and there’s only about 20% improvement from 12 weeks to 4.5 months. This helps us translate some of the other results we’re seeing into longer term impact; most of these results are reported at 5 weeks or 12 weeks after infection.
[Note that another study claimed that the rate of long-term symptoms in symptomatic COVID patients dropped from 13.3% at 4 weeks to 2.3% at 12 weeks, which would imply that only 17% of symptoms present at 4 weeks are still present at 12 weeks. This study is older and has a much smaller sample size entirely from early in the pandemic and generally appears to be an outlier relative to some other studies, so I'm less inclined to pay attention to its results.]
To get a good picture of what happens after more than 4.5 months, we'll have to look at the data from other coronaviruses such as SARS and MERS. These diseases were on average more severe than COVID, and hit in the 2000s back when PCR tests were harder to come by, so all the studies I found on them only looked at hospitalized patients as opposed to milder cases. That said, here's what we can learn:
- A recent systemic review and meta-analysis of long-term SARS and MERS studies that was specifically conducted to look for analogies to long COVID showed lasting impact several years after infection, with only mild improvement between patients less than 6 months after infection and patients more than 6 months after infection. That said, the authors acknowledge it's hard to compare data between different studies with different methodologies.
- Another study that looked at lung CT data in the same set of hospitalized SARS patients over a 7-year period showed that there was usually mild to moderate improvement from 6 months to 7 years, only one of the 11 patients studied was clear of abnormalities after 7 years.
- Another longitudinal study that tracked a wide variety of physiological and mental performance markers in the same set of SARS patients over the 24-month period after infection (3,6,9,12,18, and 24 months) showed that there was not a clear pattern of improvement over this period. This was true both for younger (<40) and older (41-64) patients. However, the study notes that a lot of the patients dropped out of the followups, and the ones that dropped out were younger and healthier than the ones that didn't. It's unclear how fully they corrected the analysis for this.
- Finally, one study looked in particular at mental issues and chronic fatigue in recovered hospitalized SARS patients. It noted that another study showed 64% incidence of psychiatric illness at 1 year post infection, and they found 40% incidence 3.5 years after infection in their own study. 64% -> 40% is not a huge improvement.
Because all of these studies are on hospitalized SARS or MERS patients, they may not generalize fully to milder cases of a different coronavirus. However, they likely give a sense that serious COVID cases probably lead to persistent negative outcomes.
We can also look at the long-term outcomes of people who are diagnosed with chronic fatigue syndrome as fatigue is one of the most common long COVID symptoms. CFS by definition is fatigue lasting more than 6 months and can come from a variety of causes. For adults who get CFS, full recovery is rare. Adolescents with CFS fare somewhat better, but a large fraction (maybe 20-50%) are still having issues years later.
In the end we need to make an educated guess, even if it's a low-confidence one, as to how often long COVID that lasts 4.5 months ends up being lifelong. Based on the SARS data, we could guess that 80% of hospitalized acute COVID patients that that have long COVID at 4.5 months end up having it for the rest of their life. Patients with milder COVID cases tend to get less physiological damage during acute infection, so it's possible they'll have higher recovery rates. Again taking an educated guess and going on even less data, we might expect that 50% of long COVID cases for mild acute patients at 4.5 months end up being lifelong.
How do age and sex affect the incidence of long COVID?
The high-quality study from the UK with the control group also has information on long COVID by age and sex. In general women are at higher risk, and young people are at reduced but not zero risk. Note that these data are for symptoms at 5 weeks, so we’ll want to multiply them by 0.55 to get the potential 4.5+ month long term rate. As before, this is prior to vaccination and prior to the Delta variant.
How does severity of infection affect the incidence of long COVID?
In general, increasing case severity leads to an increased chance of diagnosis with long COVID. Being hospitalized greatly increases the chance of long COVID diagnosis, and ending up in an ICU has an even greater impact. This is great news (at least for vaccinated people), as vaccination tends to greatly reduce the severity of the acute disease. However, the rate of occurrence of at least one long COVID symptom surprisingly might not be that different for asymptomatic vs symptomatic but not serious (not requiring hospital admission) cases. The FAIR study counts long COVID as doctor visits for a new condition starting as soon as 30 days after infection, and noted a 19% occurrence for asymptomatic patients vs 27% for symptomatic patients and 50% for hospitalized patients. Note that these rates are at ~4 weeks, so we’ll want to roughly cut them in half to estimate the frequency of very long term (4.5+ month) effects. Even then, the result that perhaps ~10% of people who have asymptomatic infections go on to develop an ongoing issue that lasts many months is shocking.
One outlier study (pre-vaccination, pre-Delta) claims there's no significant relationship between acute disease severity and the severity of long COVID respiratory and fatigue symptoms, though there appear to be some biases in how the patients were collected for the study that might lean toward patients who were in poor health in general, and their collection dates likely excluded most asymptomatic cases.
One study from the UK looking just at cognitive deficits (pre-vaccination, pre-Delta) noticed significant cognitive declines in people who had recovered from COVID. This study shows a strong relationship between severity of COVID and the average amount of cognitive decline, with hospitalized patients suffering about 3x more than symptomatic-but-not-needing-medical-assistance patients and 6x more than asymptomatic patients.
The study tries its best to correct for all extraneous variables, such as the possibility that people who get more severe cases of COVID have more comorbidities to begin with, but it’s still dependent on people who took the Great British Intelligence Test online either for fun or because they were worried about cognitive decline, which introduces unavoidable bias into the results. There is however another study from the UK that has brain scans of the same people before and after COVID, and it shows a loss of gray matter in certain areas following COVID, so it's likely that the cognitive decline effect seen in the other study is real.
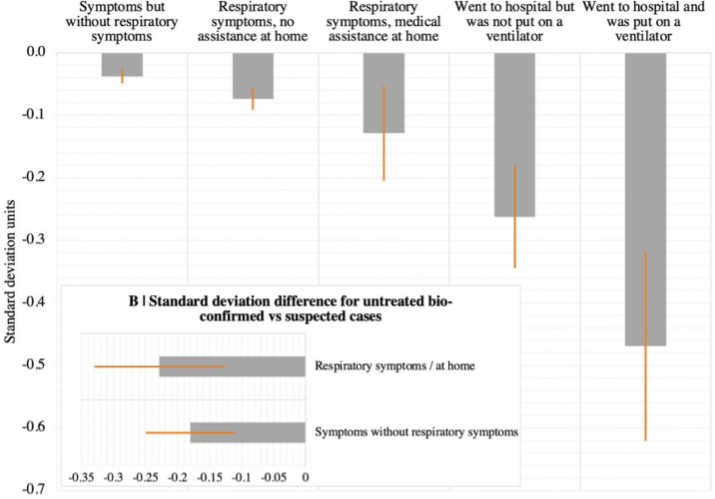
This research suggests a stronger relationship between initial case severity and the incidence of long COVID than the FAIR study did, at least for mental effects.
Note that this is the average decline – if there’s a distribution of mental effects in the same way that there’s a distribution of physiological effects, we could be seeing a situation where most people see no cognitive decline but a few see a large decline. It's fortunate that the vaccine dramatically reduces the risk of hospitalization even though it only has a more moderate impact on getting COVID at all, so vaccinated people are spared a larger fraction of the cognitive decline risk.
In review, we have two major studies looking at the impact of acute disease severity on long COVID risk, with one showing hospitalized COVID patients having roughly 2x the long COVID risk as symptomatic patients and 2.5x the risk of asymptomatic patients, and another showing hospitalized patients having 2-3x the long-term mental impact from COVID as symptomatic patients and 6x the impact as asymptomatic patients.
How common are different symptoms in long COVID?
Here's a very detailed chart comparing the occurrence of a wide variety of post-COVID conditions broken down by different levels of acute disease severity, as gathered from VA medical records in the US. The three groups include people who were COVID PCR positive but not hospitalized (everything from asymptomatic to serious but not requiring hospitalization), hospitalized (but not admitted to the ICU), or admitted to the ICU.
Note that the left side “hazard ratio” is the relative increased chance of the condition vs its rate in the general population, whereas the right side “excess burden” is the absolute number of appearances of this symptom over baseline per 1000 patients. Thus, issues like hair loss and smell problems have a large relative increased risk but a small absolute increased risk, whereas issues like fatigue and shortness of breath are a large increased risk in both a relative and absolute sense.
As we saw in the previous section, we can see there is a relationship between case severity and the severity of long COVID symptoms.
How debilitating is long COVID?
In order to understand how much we should look to avoid long COVID, we need to understand how bad it is. The UK did a nationwide survey of long COVID sufferers in March 2021 to understand how much long COVID affected their daily activities. Here's what they learned:
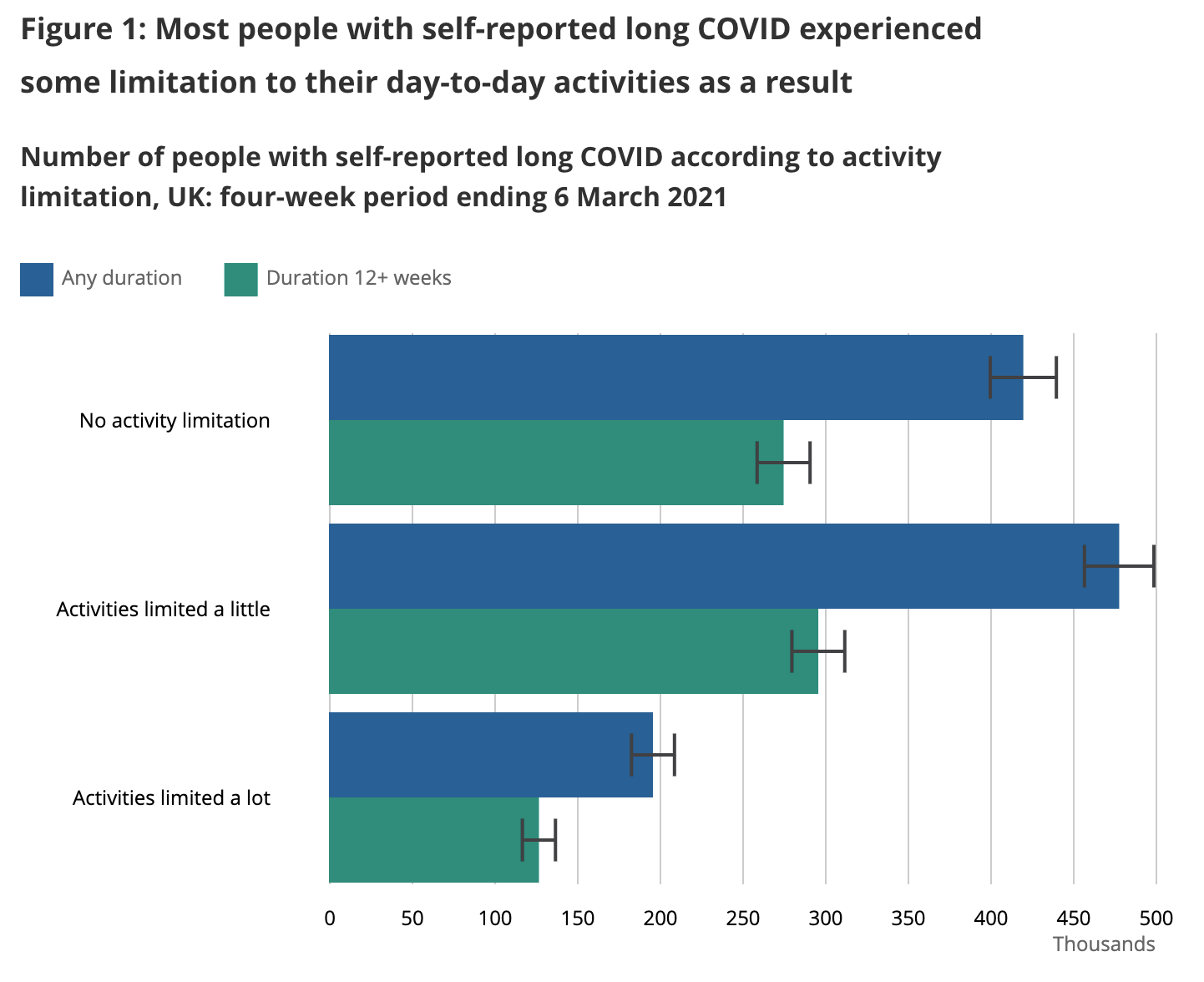
We want to come up with an estimate of how much each outcome affects quality of life so we can decide how hard we want to work to avoid long COVID. An X% quality of life reduction should be equivalent to an X% shorter life – eg you should be equivalently unhappy with a 50% quality of life reduction vs living 50% fewer years.
The question on activity limitations was specifically written as:
“Does this reduce your ability to carry-out day-to-day activities compared with the time before you had COVID-19?”
Possible responses comprise “Yes, a lot”, “Yes, a little” and “Not at all”.
Thinking about these responses:
- I’d guess “Not at all” probably corresponds to symptoms like loss of taste or smell, hair loss, persistent cough etc. I’d assign a ~2% quality of life loss to this outcome, though it’s sad to imagine not enjoying the taste of meals again.
- I’d guess “Yes, a little” probably corresponds to symptoms like headache, joint pain, some sleep disorders etc, where they’re a constant daily distraction and a limit on your level of physical exertion but you can still do most things. I’d assign a ~15% quality of life loss to this outcome.
- I’d guess “Yes, a lot” probably corresponds to symptoms like serious fatigue, tachycardia, memory problems etc where you can no longer hold down a job, it’s hard to simply walk around the house, let alone a city or a nature trail. I’d assign a ~60% quality of life loss to this outcome. Here I'm baking in a bit of a length-of-life reduction into the 60% as well, as this level of damage generally shortens life.
In just the last week, the UK released new data where they broke out the long COVID sufferers by how long ago they had COVID. This is exciting because we finally get a peek into what long COVID looks like for people who have had it for more than a year. Here is just that group:
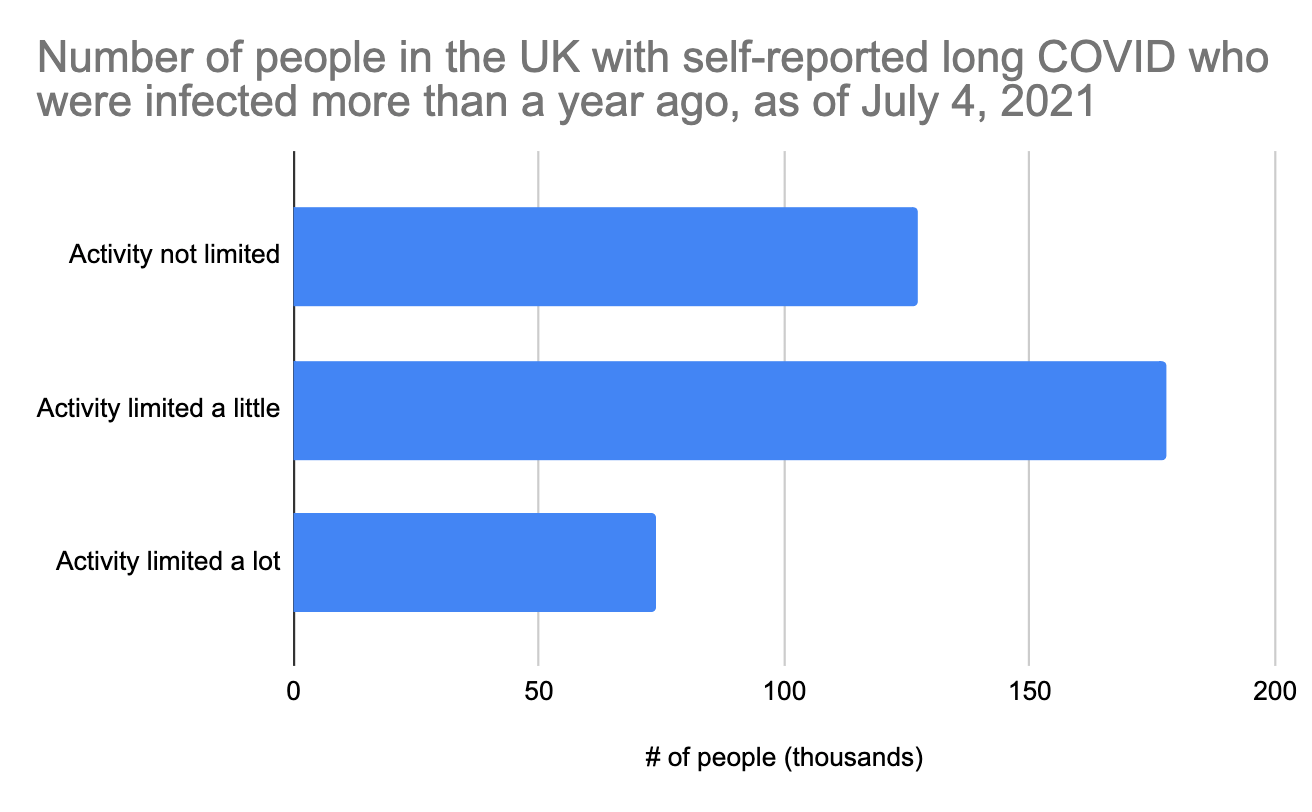
The distribution is different from the 12-week long COVID graph above, but not radically different. Note that these people were all infected during the first wave in March-June 2020. Unfortunately, COVID testing during this period was not good at all, so we don't really know how many COVID cases this represents, and we can't use it to estimate what percent of people have long COVID after a year. The UK does have good data on hospitalizations from that period though, and it looks like there were about 240,000 prior to July 2020, the period reflected in the above graph. Since only a fraction of hospitalized acute cases develop long COVID (we earlier estimated 25% at 4.5 months), we could roughly estimate that maybe 60,000 of the above people were hospitalized, which means that most of these long COVID cases are coming from people who were not hospitalized.
The new UK dataset also breaks out the data on long COVID severity vs initial case severity. This is exciting as we finally get to start seeing how the vaccine's reduction in disease severity starts impacting the severity of long COVID.
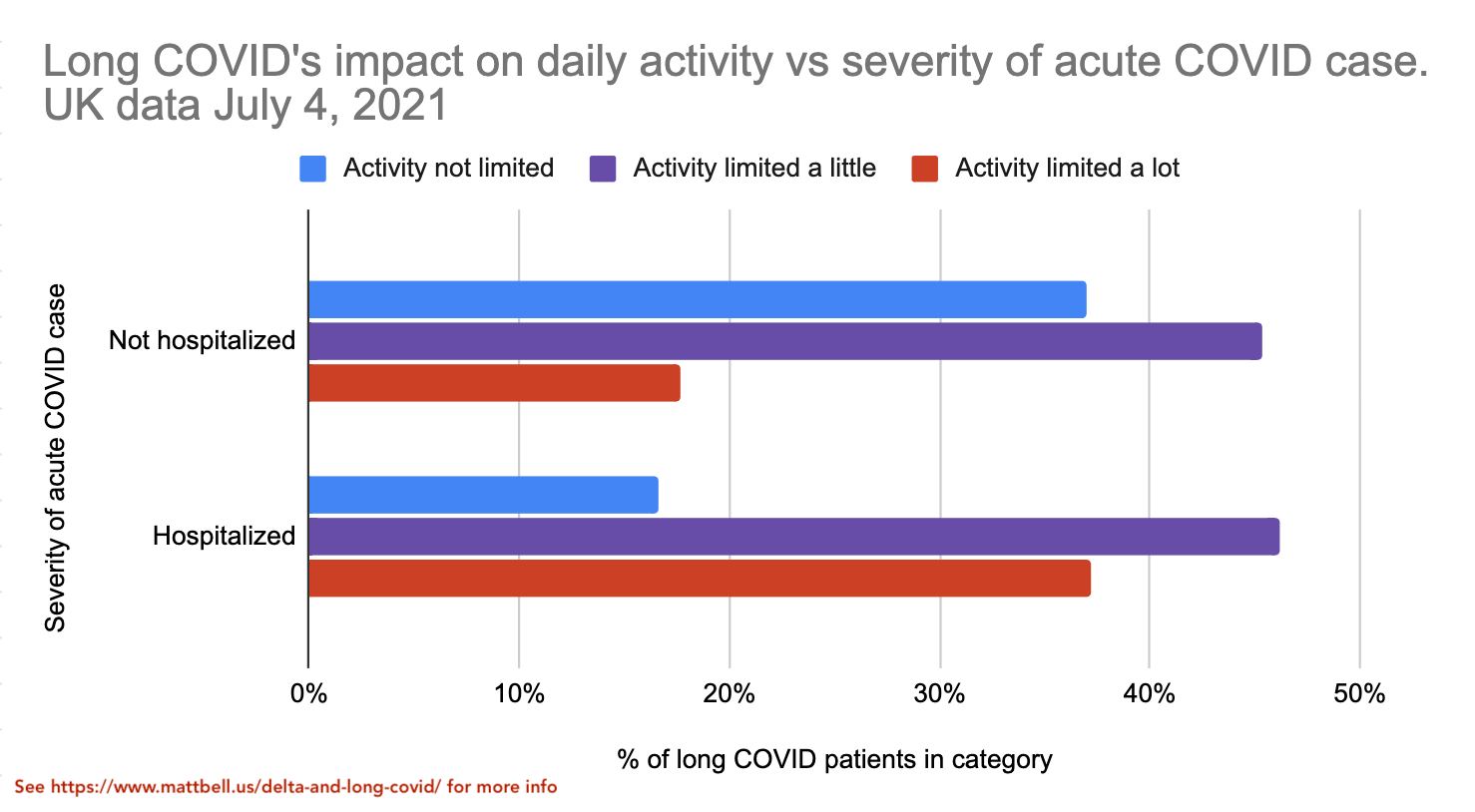
It's good to see that the "activity limited a lot" category is significantly less common for the non-hospitalized group, though it's still significant at ~18%.
Now we can arrive at an estimate of the expected quality of life reduction from getting long COVID from a non-hospitalized case of COVID. We learned earlier that the activity limitation distribution of the long COVID cases doesn't change that much with the length of long COVID, even as some people do get completely better. Thus, we can assume that the data for "Not hospitalized" and "Hospitalized" groups above will likely have a similar distribution with 1+ year long COVID as they do with shorter-term long COVID. Then, based on quality-of-life numbers mentioned earlier (2% for no activity limitation etc), we can estimate that the time spent with long COVID has on average a 18% quality of life reduction for non-hospitalized acute cases and a 30% quality of life reduction for hospitalized acute cases. Again, if you're vaccinated you're almost certain to have a non-hospitalized acute case, whereas if you're not vaccinated you have significant risk of being hospitalized.
Early evidence for the impact of vaccination on long COVID:
As mentioned earlier, there still isn’t good data on how mRNA vaccination prior to catching COVID impacts the incidence of long COVID from Delta.
A very recent report from the UK claims that full vaccination cuts the risk of lingering symptoms 28+ days after catching COVID by roughly half. However, this was based on data from the UK that primarily reflects people vaccinated with the weaker AstraZeneca vaccine getting infected with the B117 variant, so results against Delta with prior mRNA vaccination may be better or worse.
Strangely, the original paper cited by this report does not show a reduction in incidence of long COVID following vaccination except for older patients, but the report and original paper have many of the same authors, so it’s likely that the original paper’s authors approve of the veracity of the above figure. Differences may be due to one-dose and two-dose vaccinated individuals being lumped together in the original paper or some other difference.
There’s a recent study of post-mRNA-vaccination COVID infections of Israeli nurses showing that 19% had symptoms 6+ weeks after infection, but their sample size for this study was only 39 cases, so it’s hard to conclude anything from this data aside from the existence of long COVID in vaccinated patients.
The survey in the long COVID support group that found 56.7% of unvaccinated long COVID sufferers reported improvement in symptoms after vaccination provides some indication of a protective benefit of vaccination on reducing the chance of long COVID. The causal mechanism for this may or may not be independent of how the vaccine reduces the chance and severity of COVID infection, and may or may not indicate a reduction in the chance of developing long COVID after vaccination. However, at the very least, long COVID sufferers could get treated with another dose of the vaccine, and there's a reasonable chance half of them would get better based on the aforementioned survey.
Based on all the above evidence, I’m making a very rough guess that mRNA vaccination cuts the risk of a Delta COVID infection developing into long COVID in half, independent of vaccination’s reduction of the risk of catching Delta at all. Note that there are many shaky assumptions here in terms of differences between the performance of different vaccines and differences between Delta and older variants like B117.
Strategies, revisited:
How can we translate this into a potential risk of long COVID for vaccinated people who catch Delta? It depends on how you behave.
If you follow strategy (1) -- the “f*ck it, I’m going to get Delta eventually, and it will just be the flu” strategy, then you can assume that you’ll get a likely mild case of COVID at some point in the near future, and the question is how much it will hurt you. Let’s see how this impacts the chance of long COVID, taking a series of estimates:
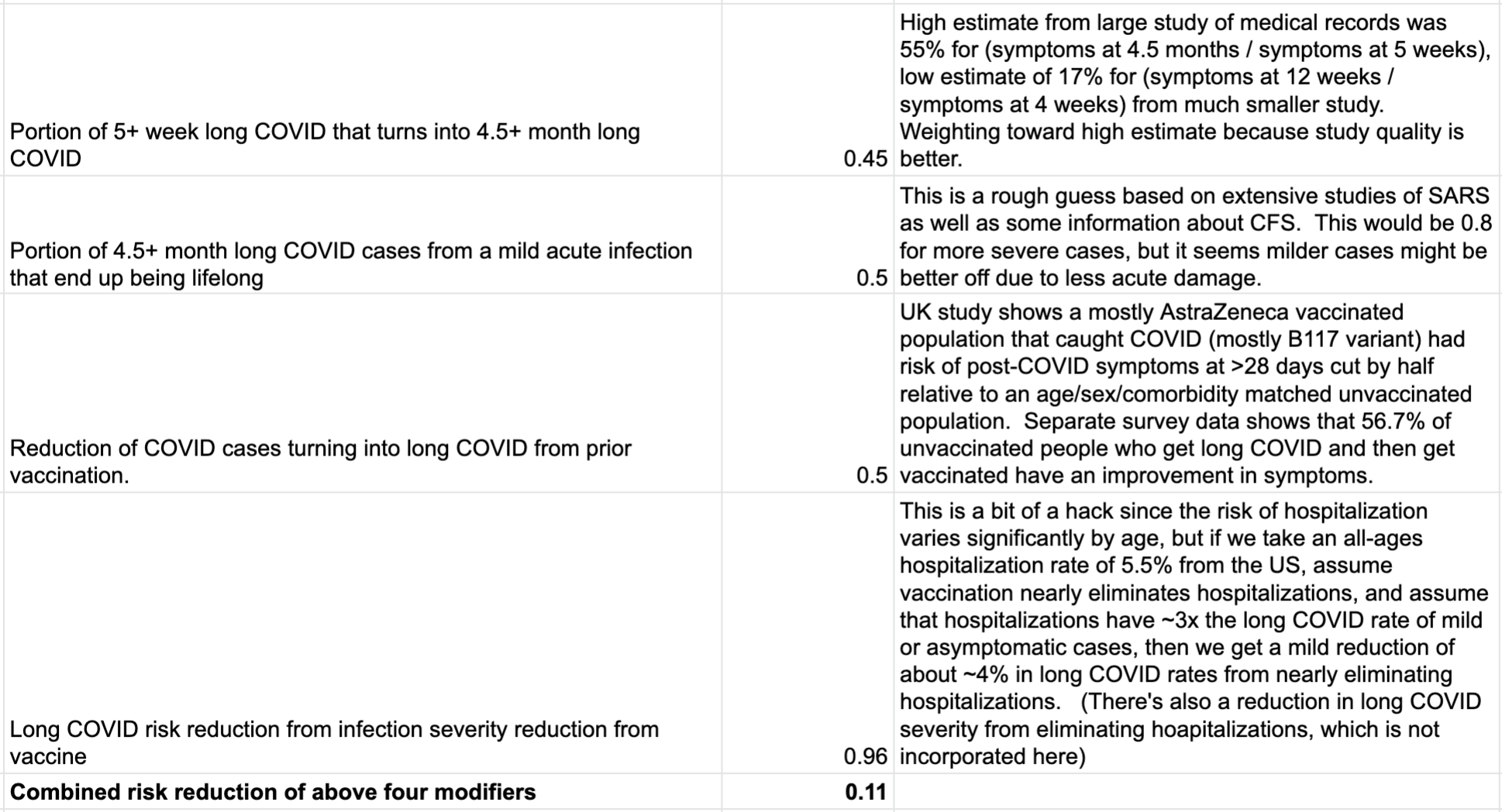
If we apply this 0.11x modifier to the risk of long COVID from the high-quality UK study, we get the following:
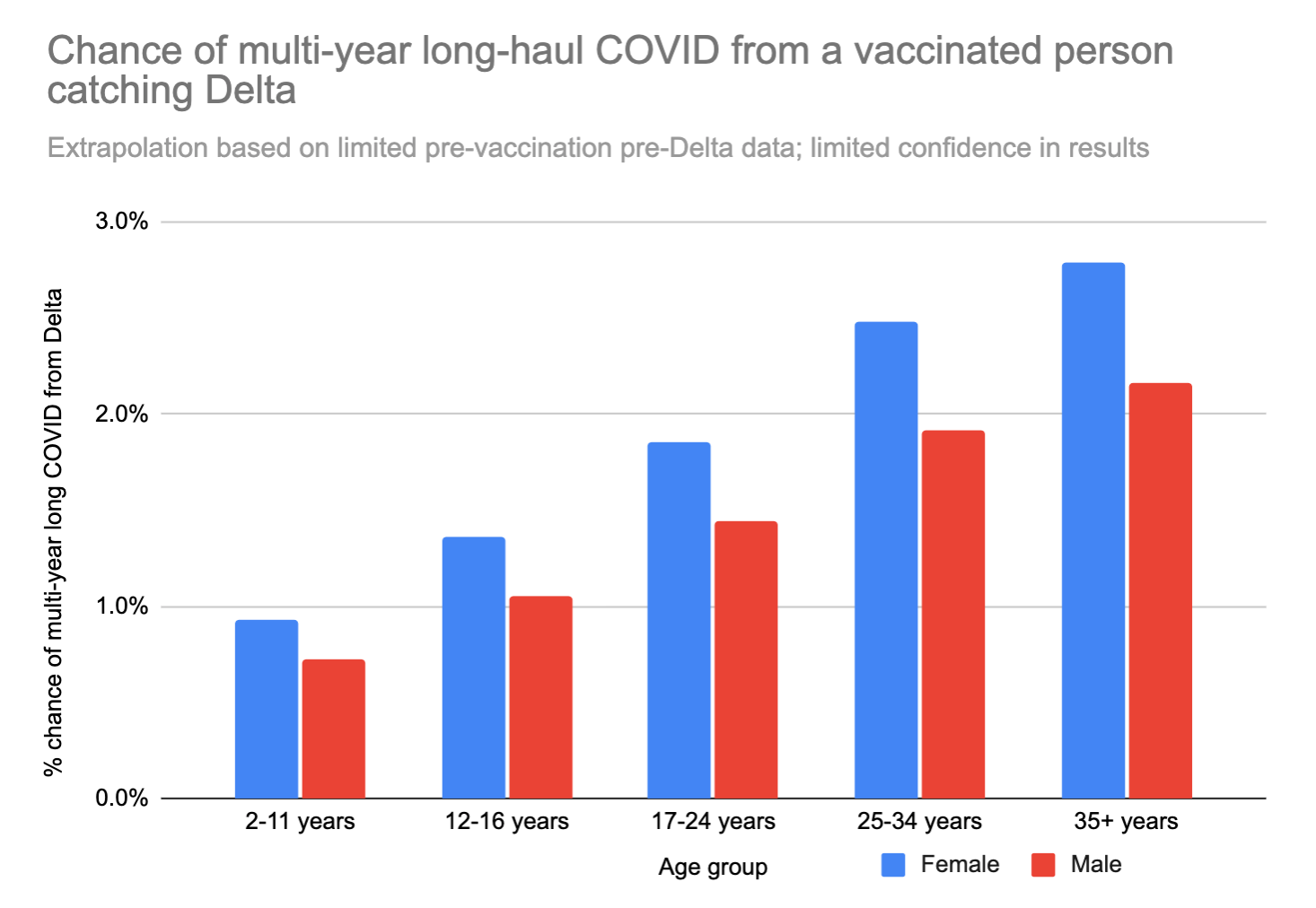
These numbers are low, but not low enough to ignore. Earlier we decided that the quality of life hit from long COVID after a non-hospitalized acute case was 18%. If you’re a 35 year old woman, and your risk of ending up with lifelong long COVID from catching COVID is 2.8%, then catching COVID would be the same, statistically speaking, as losing (50 years * 0.18 * 0.028 * 365 days/year) = ~90 days of your life. Ouch.
We can also look at just the "worst case scenario" – catching long COVID that doesn't go away for years AND limits daily activities a lot. This number feels a bit more like a "mortality" rate – except in this case you don't actually die, but your life is forever altered, and you can't hold down a job anymore or do most of the things you used to love to do.
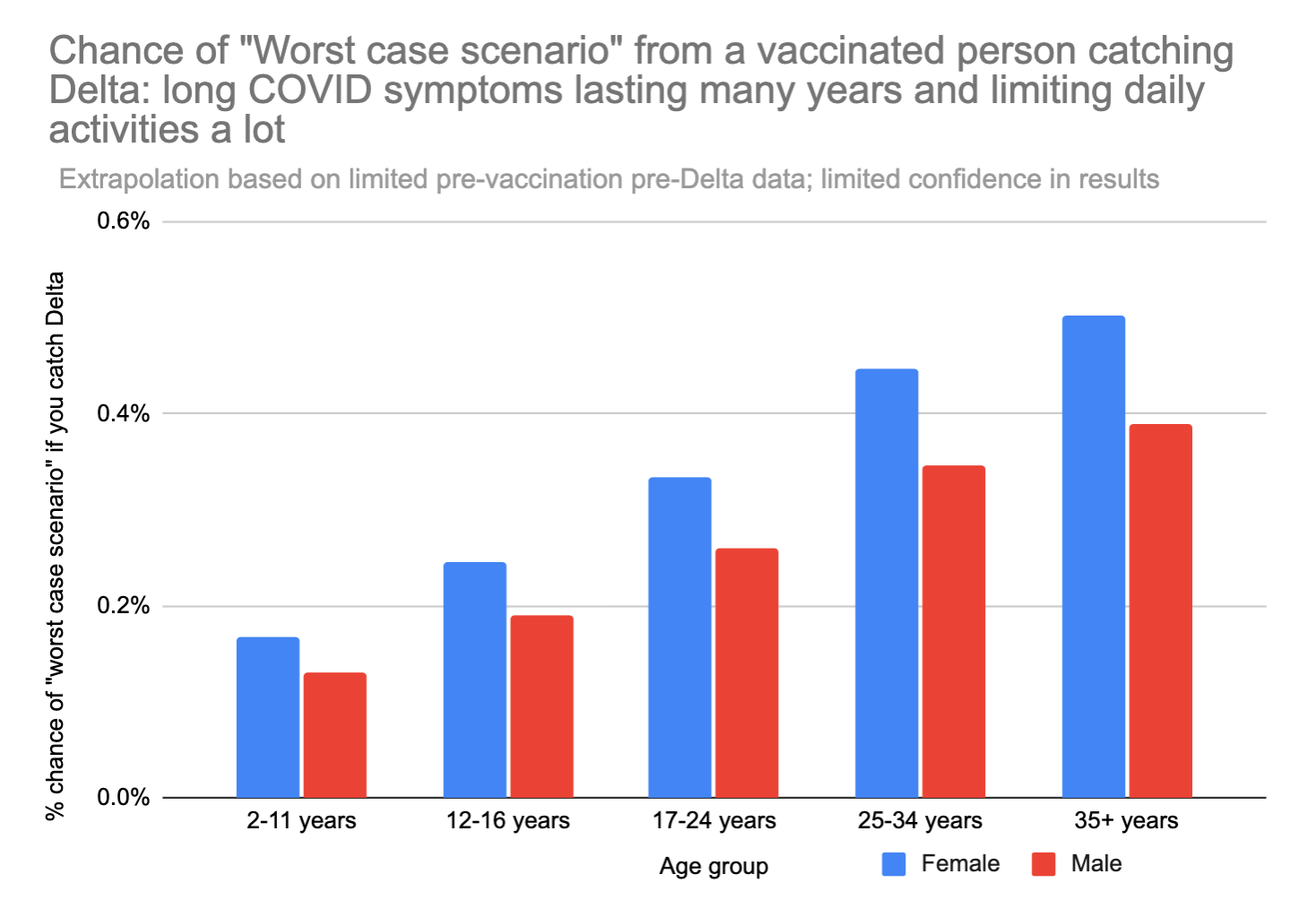
A 35 year old woman runs about an 0.5% chance of the "worst case scenario" outcome if she gets Delta. For comparison, 0.5% is about 42x your chance of dying in a car crash in the next year.
On the other hand, what’s the cost of avoiding several months of social activities to avoid catching COVID until the rest of the country reaches herd immunity or booster shots are available? It’s hard to say, and it probably depends on your risk tolerance and how bad the last year of restricted socializing felt for you. There's also the option of being extra careful just during the peak in Delta caseload, which might only last 2 months before subsiding.
The expected loss from catching COVID might also be somewhat of an overestimate, as not everyone will catch Delta over the next few months. Variations in natural immunity and the boost from vaccination create significant individual variation in susceptibility to COVID, and not everyone will catch COVID before herd immunity is reached. Indeed, the UK’s Delta caseload has already peaked and is declining, so it looks like it will not come close to infecting everyone. The official numbers suggest that just the Delta wave has infected about 2.5% of the population so far, but it's likely the real number is substantially higher due to underreporting of mild cases.
If you choose strategy (2) and reduce your exposure by taking precautions such as avoiding unmasked indoor gatherings, you’ll get a big additional protection from long COVID from the vaccine’s ~64% reduction in infections. Stacking with the other reductions noted above (except the multipliers for long COVID length), a fully vaccinated person taking good precautions might be at a ~1/6x the risk of developing long COVID relative to an identically behaving unvaccinated person.
If you’re using microcovid.org, the above information should help you decide what risk of catching Delta you’re willing to take on in the next year. As someone who was on the “1% annual risk of COVID” budget prior to vaccination for both personal and social responsibility reasons, the above information leads me to be willing to take on a risk budget of a 10% annual risk of catching COVID, primarily out of a fear of getting some debilitating physical or mental condition from long COVID, and thus being willing to accept a ~7 day hit in quality-adjusted lifespan per year of operating under this budget. Going back to the car crash comparison, I'll be a bit over 3x more likely to get the "worst-case scenario" long COVID this year compared to my chance of dying in a car crash this year.
However, many of the above numbers are based on shaky projections that could swing significantly when preliminary data on long COVID rates from the Delta variant are finally available, which could be as soon as the next week or two. It might be worth being more cautious for a few weeks until this data is available and the current spike in Delta cases in the US passes. I'm personally aiming at a 5% annual risk of catching COVID instead of 10% as described above in the short term because of this uncertainty.
Hopefully this early analysis, albeit imperfect and based on limited data, is useful to you.
Thanks for proofreading, information, and feedback from Elizabeth Van Nostrand, Lily Dorman-Colby, and everyone at the microcovid team who took a look at this. Views expressed here are mine alone and do not represent a team consensus from microcovid.
